Detectable 8 driver genes,
74 DNA mutations in one well
产品料号:82030
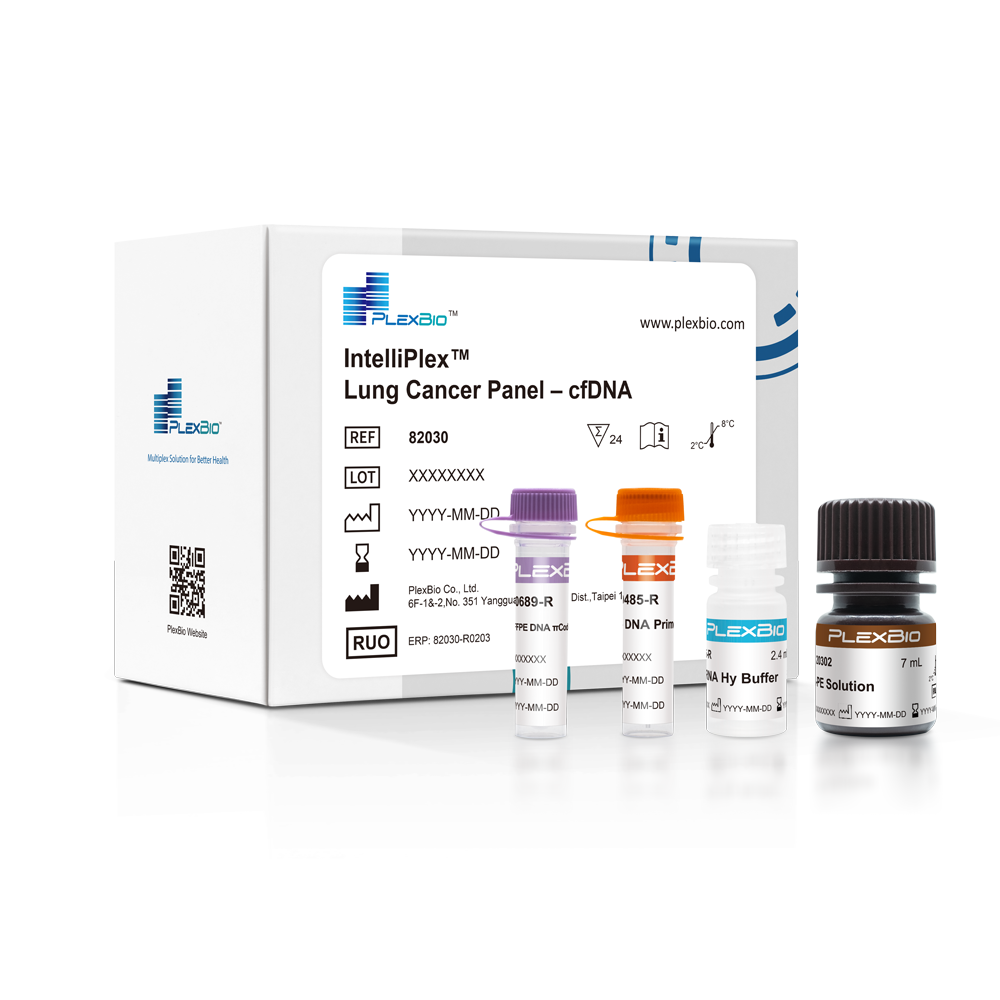
IntelliPlex™ Lung Cancer Panel - cfDNA
The IntelliPlex™ Lung Cancer Panel comprises NSCLC driver genes' DNA mutations and RNA fusion variants detection for use with samples extracted from NSCLC formalin-fixed paraffin-embedded tissues (FFPET) or liquid biopsies such as plasma. The cfDNA panel is a qualitative molecular assay for the detection of 74 DNA mutation hotspots in the KRAS, NRAS, PIK3CA, BRAF, EGFR, HER2, MEK1 and AKT1 genes from plasma samples in a single well by using PlexBio's core πCode™ technology.
FEATURES
FEATURES
- Detect 8 driver genes, 74 mutation hotspots in one well test
- Highly sensitive (0.1~2.32%) to detect low levels of mutant DNA
- Only ≥10ng DNA sample required
- Time to results in <6 hours for 96 samples per run (sample preparation included)

Broad Target Coverage

Low Sample Input
Only 10ng DNA sample extracted from FFPE tissues required

High Sensitivity
Detects low levels of mutant DNA with a high sensitivity

Quick Turnaround Time
Sample to results in <6 hours with automated reporting
Overview
The IntelliPlex™ Lung Cancer Panel- cfDNA is an in-vitro molecular test based on πCode™ technology and PlexBio's instrument platform to identify 74 DNA mutations in KRAS, NRAS, PIK3CA, BRAF, EGFR, HER2, MEK1 and AKT1 genes in NSCLC. πCode technology enables the multi-mutations detection in single-well with DNA samples derived from plasma. The kit provides all necessary optimized reagents for the sensitive detection of low levels of mutant DNA in a background of wild-type genomic DNA by simultaneous PCR amplification of target mutations. The genetic status evaluation from these NSCLC driver genes can impact the selection of and the response to targeted therapies.
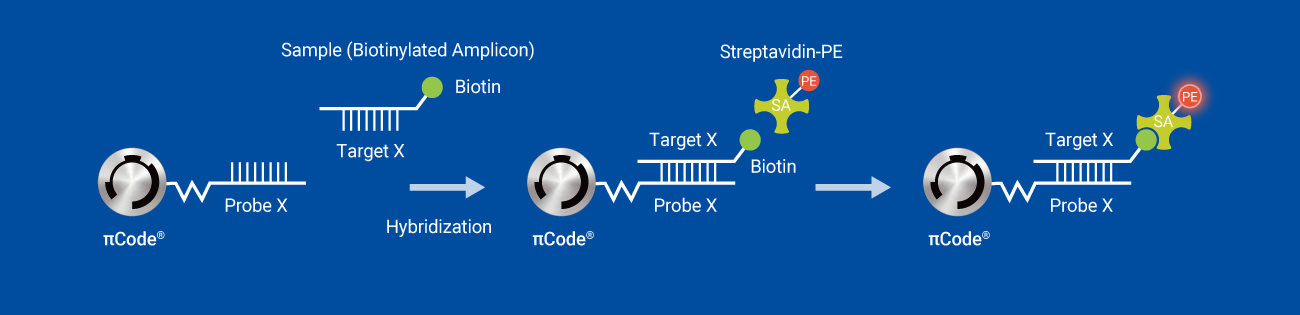
Principle of πCode Multiplexing
OUR RESOURCES
IFU